E&L Risk Assessments
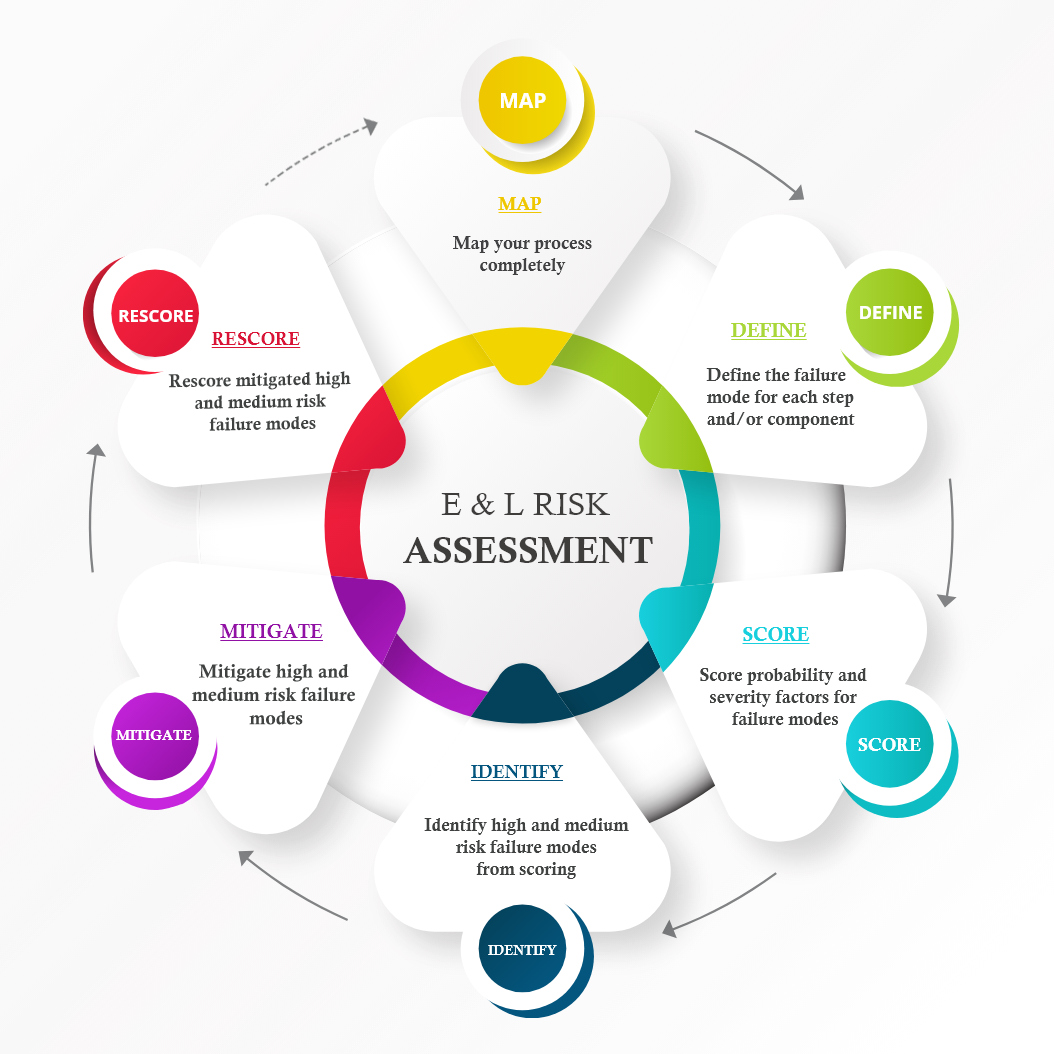
Working with client subject matter experts, ExtLe Solutions Limited designs, manages, and executes Extractables and Leachables Risk Assessments, using Failure Mode and Effect Analysis (FMEA).
Failure modes associated with leaching are identified for the clients manufacturing process and/or container closure system. Either of these can apply to API, BDS or drug product. Each failure mode is assessed for the likelihood (probability) of leaching occurring against what the severity would be if leaching occurred, allowing each failure mode to be classed as low, medium or high risk.
The FMEA process results in a tailored assessment of what risk leachables pose to the clients final drug product, by defining the materials most at risk of generating leachables during manufacture and/or shelf life storage. As such, the risk assessment informs on which materials require E&L studies, and which do not. Additionally, it provides an opportunity for other mitigation activities to be defined for higher risk areas of the manufacturing process, packaging and container closure system.
Ready To Talk?
If you're ready to discuss your requirements and would like to arrange a call with us click the button below, otherwise if you have general questions or comments please don't hesitate to get in touch
Registered in England No. 10155666 | VAT No. 246 7451 88
© 2021 ExtLe Solutions Limited. All Rights Reserved
View our Privacy Policy